ROLE OF PROCESS VALIDATION IN PHARMACEUTICAL INDUSTRY: AN OVERVIEW
Abstract
Author(s): Maurya Jyoti
Drugs are the critical element in health care. They must be manufactured to the highest quality level. End product testing by itself does not guarantee the quality of the product. Quality assurance technique must be used. In pharmaceutical industry, process validation performs this task, ensuring that the process does what it purport to do. Validation is one of the important steps in achieving and maintaining the quality of the final product. If each steps of production process is validated we can assure that the final product is of the best quality. Process validation also emphasizes the role of objective measures and statistical tools & analyses and emphasizes knowledge, detection, and control of variability and gives assurance on consistent of quality/productivity throughout life cycle of product. Validation is the tool which establishes a high degree of assurance that all processes meet their intended specifications. Hence pharmaceutical validation becomes significant in pharmaceutical industry. This overview summarizes the various aspects of pharmaceutical validation. This paper represents an introduction and general overview of process validation of pharmaceutical manufacturing process.

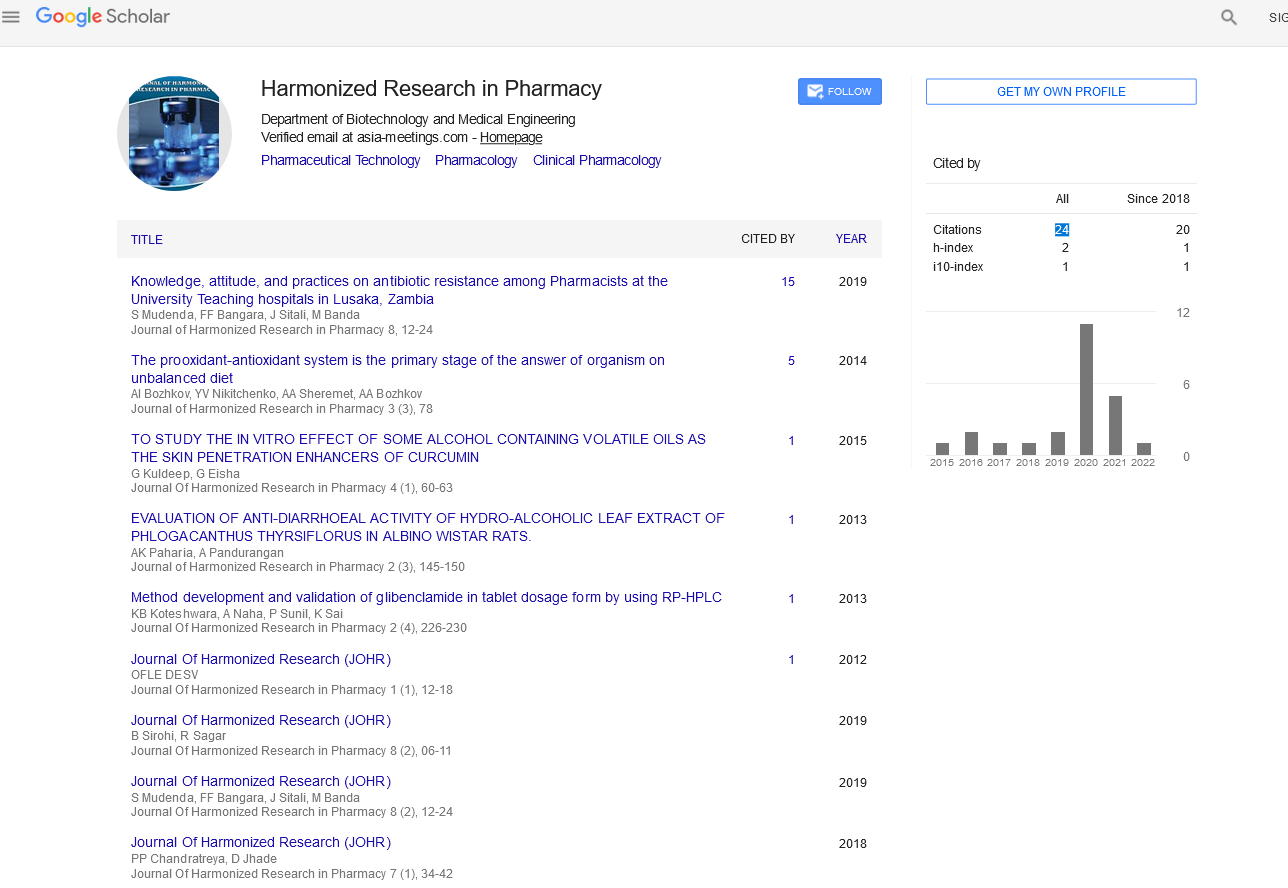
Google Scholar citation report
Citations : 147
Journal of Harmonized Research in Pharmacy received 147 citations as per google scholar report